GORE® STA-PURE® Flexible Freeze Container
Single-use GORE® STA-PURE® Flexible Freeze Containers are intended for storing and transporting bulk drug substances after freezing at -86°C (-123°F).
GORE® STA-PURE® FLEXIBLE FREEZE CONTAINERS MINIMIZES RISK OF PRODUCT LOSS AND CONTAMINATION THROUGHOUT THE COLD CHAIN
A Bioplan survey found that nearly 75% of biopharmaceutical manufacturers considered bag breakage, product loss, leachables and extractables, as problems that limited their ability to use disposable solutions.
Single-use storage containers offer numerous benefits to biopharmaceutical manufacturers and are widely utilized in drug production. However, traditional polymer-film-based disposable bags have certain vulnerabilities:
- They can become brittle and fragile at low temperatures, increasing the risk of damage and product loss during cold chain handling, transport, or storage.
- Extractables and leachables from the materials used in disposable packaging pose potential concerns.

Impact Performance: GORE STA-PURE Freeze Bags vs Competitors
Technical Specifications
GORE STA-PURE Flexible Freeze Containers are sold as an assembly and are suitable for both plate and blast freezers in different bag volumes. Maximum fill volumes vary by freezing method. Contact us for tubing and connector options.
| Volume | Dimensions (W x L) | Freezing Method | Maximum Fill Volume |
|---|---|---|---|
| 50mL | 5 x 6 in (12.7 x 15.2 cm) | Suitable for plate or blast freezers | 50 Milliliter (mL) |
| 2.5L | 8 x 17.5 in (20.3 x 44.5 cm) | plate blast | 2.5 Liter (L) 2.5 Liter (L) |
| 5.0L | 12 x 18 in (30.5 x 45.2 cm) | plate blast | 4.25 Liter (L) 5.0 Liter (L) |
| 12L | 25 x 18 in (63.5 x 45.2 cm) | plate blast | 10 Liter (L) 12 Liter (L) |
Additional Protection - Barrier Wraps
Gore offers patent-pending single-use hard-shell carriers made from durable high-density polyethylene (HDPE). These carriers, compatible with both blast and plate freezers, are optional accessories for the Container. They are designed to optimize freezer space, simplify handling, and provide added protection for tubing in the Container Assembly. Request our Use Guidelines for additional details about the carriers.
For applications where carbon dioxide (CO₂) or oxygen (O₂) permeation is a concern, an optional vacuum-sealable Barrier Wrap is available. This wrap fully encloses the Container Assembly, including its tubing, to minimize gas ingress. For more details about the wraps, request our Use Guidelines.
Sterilization
Freeze Container Assemblies are shipped sterilized and ready for use, packaged inside two TYVEK® pouches. Contact us for further information on sterilization.
At Gore, we are committed to delivering products that perform as promised. Our rigorous product testing evaluates performance both at the material level and in the final product form.
We subject our filled containers to extensive durability tests, including impact testing and multiple freeze-thaw cycles, to ensure they can endure the challenges of frozen transport, handling, and storage.
Containers filled with phosphate-buffered saline solution were frozen under two conditions:
- In a blast freezer for at least 24 hours at -86°C (-123°F).
- In a plate freezer for at least 4 hours at -70°C (-94°F).
After freezing, the containers underwent tests for frozen impact durability, five freeze-thaw cycles, and long-term storage reliability.
STA-PURE Durability Testing
| Test Performed | Testing Criteria |
|---|---|
| Frozen Impact Durability | Frozen and dropped from height of 3 feet (91.4 cm) onto concrete floor |
| Freeze/Thaw Cycles | Stored in a freezer and thawed in water bath for 5 cycles |
| Long-Term Frozen Storage | Stored for 12 months |
After testing, the containers were integrity tested by vacuum decay and visual inspection. All samples passed.
Materials Testing
The GORE STA-PURE Freeze Container is made from durable, high-purity materials specifically designed for bioprocessing applications. All container and port fluid-contact surfaces, are constructed from a proprietary 100% fluoropolymer composite. This film is chemically inert and highly compatible with a wide range of fluids.
When compared to traditional single-use bag films, the material in GORE STA-PURE Freeze Containers demonstrated a significantly lower extractables profile.
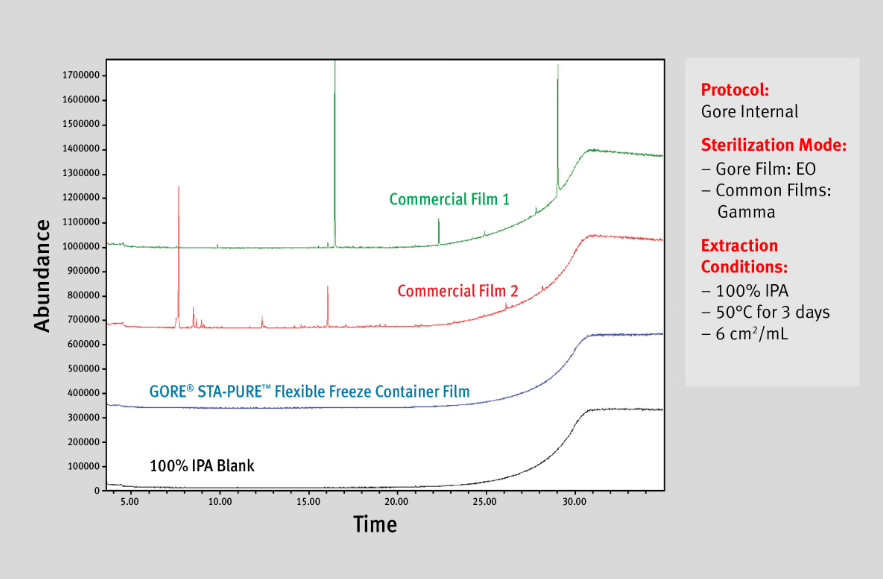
Test Details: Following the recommended sterilization protocols for each, a 6cm2/mL sample of Gore film and commonly-used films were tested in 100% IPA at 50°C for 3 days. The impurities were measured using GC/MS.
The extractables data shown is based on Gore’s internal test protocol. In line with industry standards, Gore performed an extractables study based on guidelines put forth by the BioPhorum Operations Group (BPOG) as stated in their User Requirements Pack. This study involved testing representative material sets after sterilization from the GORE STA-PURE Flexible Freeze Container’s product portfolio. Please contact us for more information about the test protocol and availability of results.
Compliance & Quality Control
GORE STA-PURE Flexible Freeze Containers (container and port) are 100% integrity tested by vacuum decay and visually inspected before quality control release. The raw materials and/or components of the fluid contact surfaces meet standards for biocompatibility, bacterial endotoxin, and particulates as shown in the table.
| Test Performed | Testing Criteria |
|---|---|
| Biocompatibility | USP ‹87› Biological Reactivity Tests in Vitro USP ‹88› Biological Reactivity Tests in Vivo Class VI |
| Bacterial Endotoxin | USP ‹85› Bacterial Endotoxin limits |
| Particulates | USP ‹788› Particulate Matter in Injections |
Gore is committed to providing our customers with rigorously compliant products that deliver high-quality performance over time. GORE STA-PURE Flexible Freeze Containers are manufactured and assembled in a manner that adheres to relevant current Good Manufacturing Practices (cGMP) as defined in the Gore PharmBIO Products' quality system, which is certified to ISO 13485 and ISO 15378. These products are manufactured following the appropriate material and regulatory requirements. Please Contact us for current compliance statements.
Explore Additional Products
Prefer to Call?
Have questions or unique requirements?
Our experts are here to guide you.
+1 800 328 4623
Have questions or unique requirements?
Our experts are here to guide you.