Gore Life Sciences Venting
Optimize your process with GORE® Microfiltration Media, engineered to enhance performance, reliability, and purity across diverse industries.
High Performance Media for Life Critical Applications
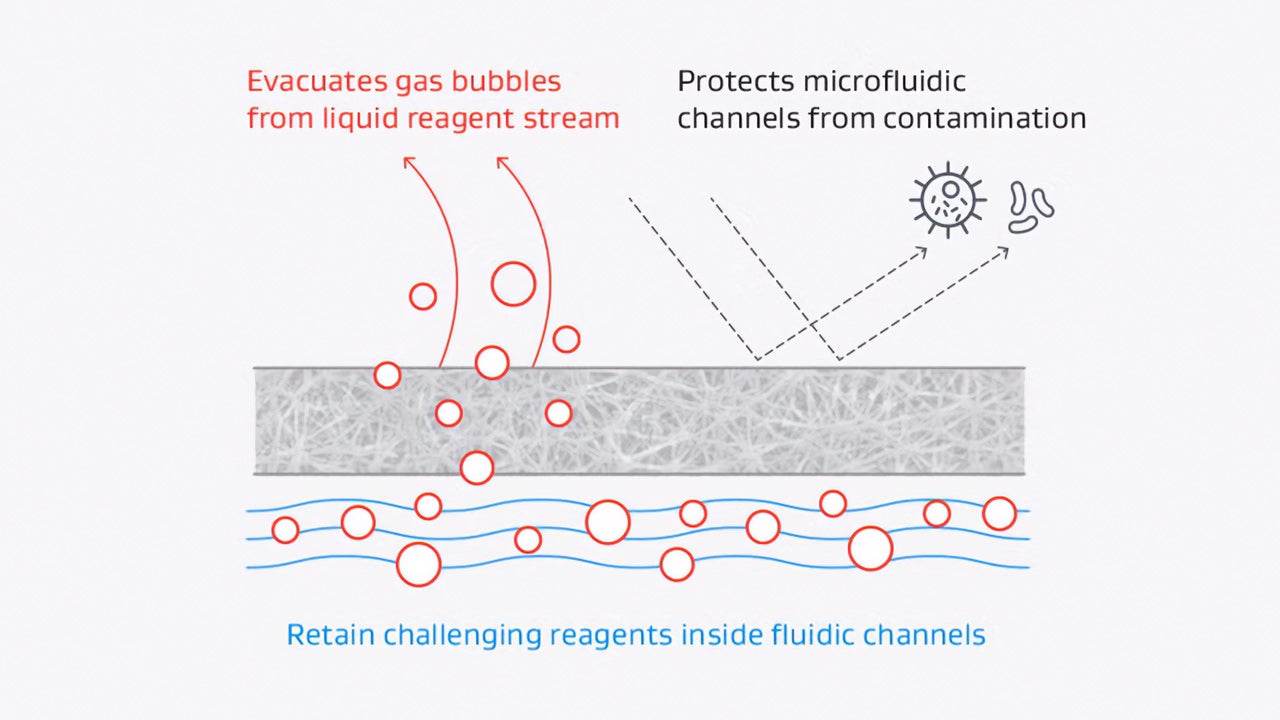
In almost all healthcare equipment applications, including bedside or wearable, venting and filtration capabilities are critical to the successful operation of the equipment. Effective venting requires high gas permeability. It is the gas permeability of the media that enables high airflow, fast pressure equalization. Filtration solutions protect equipment from contamination during use by enabling gas to pass through with low pressure drop while providing a barrier to liquids, aerosolized liquids, microbes, and particles.
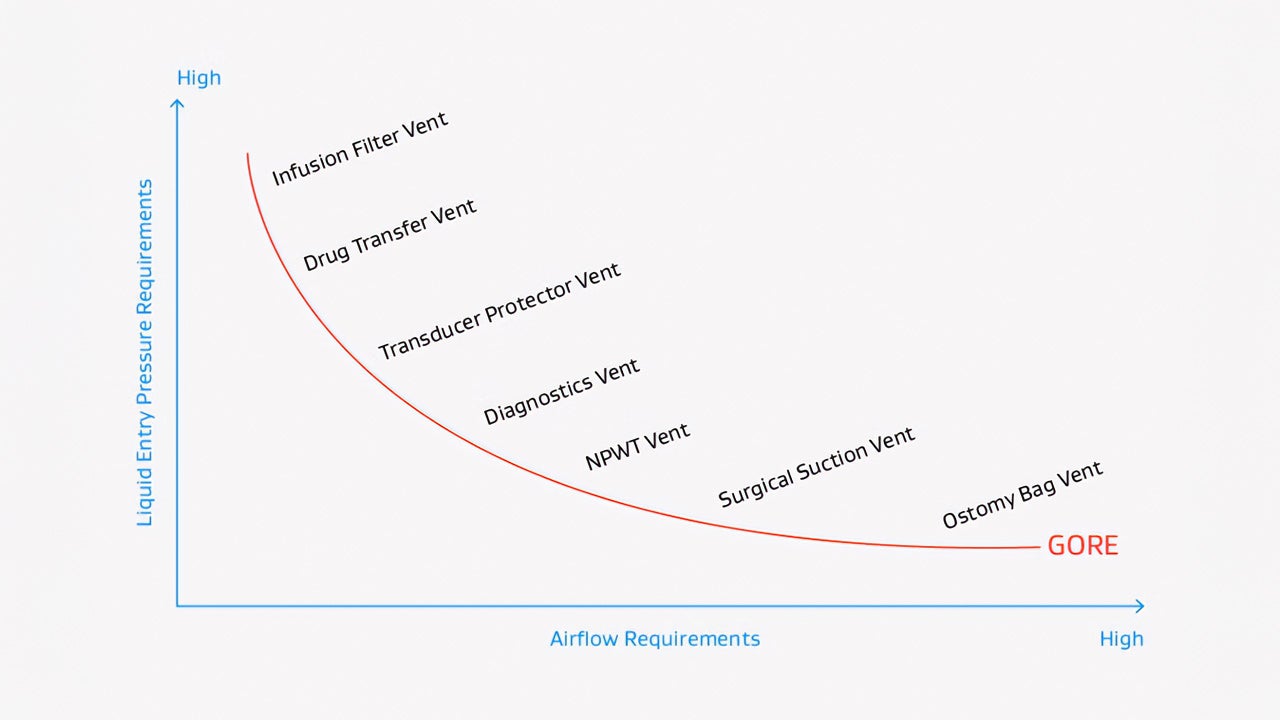
Gore has been developing high performance media including expanded polytetrafluoroethylene (ePTFE) membranes to solve the design challenges faced by device engineers for more than four decades. We are not just a supplier of membranes — we are a trusted partner that collaborates closely with device development teams to design a solution that addresses the unique challenges of your device and end-use environment. Based on individual requirements, Gore engineers will assist in the selection of the best material for our customer’s applications based on various material properties, such as density, porosity, thickness, and surface characteristics.
Explore More
Prefer to Call?
Have questions or unique requirements?
Our experts are here to guide you.
+1 800 328 4623
Have questions or unique requirements?
Our experts are here to guide you.