GORE® IMPROJECT® Plungers
Eliminate silicone in both plungers and barrels* to protect silicone-sensitive drug formulations and reduce the risks associated with particles and other with silicone-induced complications in ophthalmic and other applications.
GORE® IMPROJECT® Plungers Offer a New Solution
Today’s complex biologics, such as monoclonal antibodies and conjugate vaccines, can be vulnerable to silicone-induced protein aggregation, particle formation and drug product precipitation.
In ophthalmic applications, complications from silicone oil include increased risk of particle counts which can lead to floaters, inflammation, and interocular pressure with repeated injections.
In addition, over time, silicone migration can impact consistency of delivery, as it may change break loose/ glide force (BLGF) and injection time to silicone sensitivity.
Manufacturers can now eliminate silicone from both plunger and barrel* in their PFS
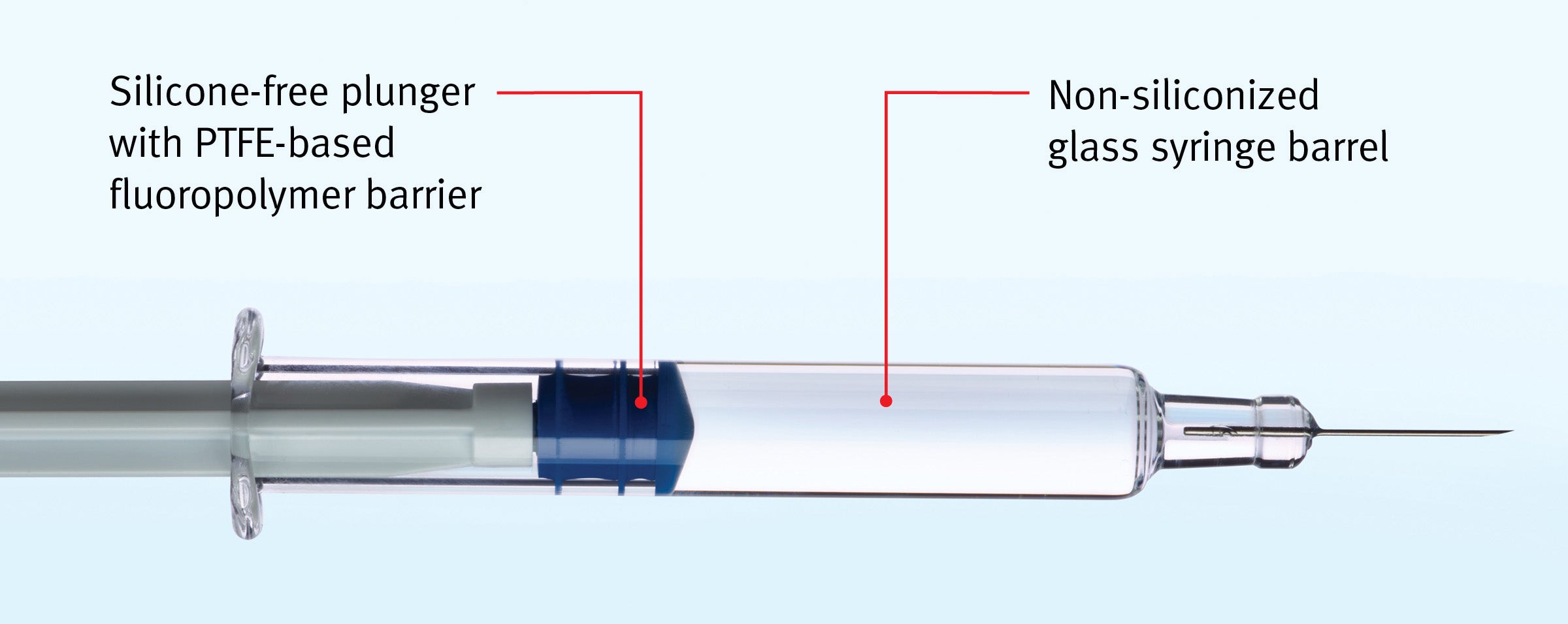
Technical Specifications
Our syringe plungers are designed to address critical challenges in drug delivery, offering unmatched performance and reliability for silicone-sensitive biologics and ophthalmic injections. With a focus on reducing particulates, ensuring container closure integrity, and maintaining consistent injection performance, these plungers provide protection against contamination and drug degradation. Their compatibility with regulatory standards and advanced manufacturing practices ensures high quality and compliance, while innovative features like low extractables and a proprietary fluoropolymer barrier safeguard drug purity. By solving industry pain points, GORE IMPROJECT Plungers set a new standard for drug delivery.
| Product Benefits | Product Testing |
|---|---|
| Exceptionally Low Particles | Sub-visible Particulate
|
| Functional Performance | Container Closure Integrity (CCI)
Reliable Performance
Extractables and Leachables
|
| High Compliance & Quality Standards | Standards Compliance
cGMP Adherence
Certified Quality Systems
|
Explore More Products
NOT INTENDED FOR USE in food contact applications or with radiation sterilization
* Not including needles or tip caps
† Silicone oil not intentionally added to raw materials or process for manufacturing plunger.
Prefer to Call?
Have questions or unique requirements?
Our experts are here to guide you.
+1 800 328 4623
Have questions or unique requirements?
Our experts are here to guide you.


