
GORE® Pharmaceutical & Biopharmaceutical
Rigorously compliant to meet the changing needs of our global customers, Gore’s solutions for pharmaceutical and biopharmaceutical manufacturers include a suite of efficient, technology-driven products for single- and multi-use applications.

Advanced Solutions for Pharmaceutical and Biopharmaceutical Processes
When patient safety and maintaining process efficiency are paramount, Gore offers a suite of high-performance products designed to meet these critical needs. Our solutions include single-use and multi-use components for drug delivery and packaging, such as the GORE® IMPROJECT® Plunger, which eliminates silicone-induced risks in pre-filled syringes.
For single-use frozen storage, the GORE® STA-PURE® Flexible Freeze Container provides robust protection against breakage at temperatures as low as -86°C. In chromatography applications, GORE® Protein Capture Devices enhance monoclonal antibody purification efficiency. Our GORE® LYOGUARD® Freeze-Drying Trays facilitate safe and efficient lyophilization processes.
Additionally, GORE® STA-PURE® Pump Tubing ensures reliable performance in peristaltic pumps under demanding conditions. With a deep understanding of fluoropolymers like polytetrafluoroethylene (PTFE) and its expanded form (ePTFE), Gore designs products that offer low extractables and resistance to chemicals and extreme temperatures, delivering reliable performance tailored to the evolving needs of the pharmaceutical and biopharmaceutical sectors.


Good Manufacturing Practices
All Gore products for life sciences are manufactured specifically for use in medical device manufacturing or pharmaceutical settings and comply with regulations our customers may require. Depending upon those requirements, you will find products manufactured under Good Manufacturing Practices (GMP), ISO 13485 and ISO 15378 certifications; and/or regulations for food contact.
Explore our solutions

Life Sciences Venting
Ensure reliable device operation by enabling venting, degassing, and liquid management, supporting high-yield production and patient safety.
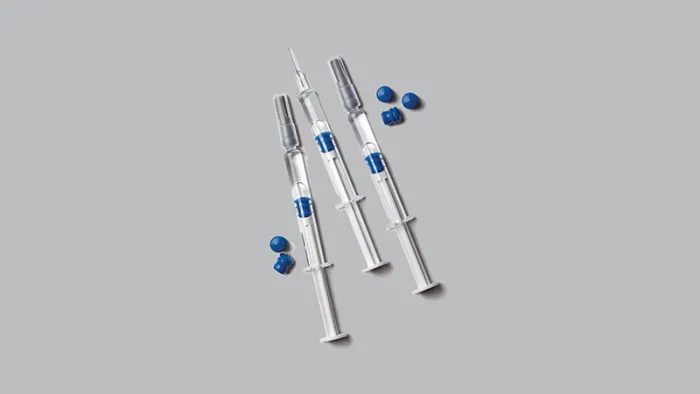
Drug Delivery and Packaging
Eliminates silicone in pre-filled syringes, protect sensitive biologics from particle formation and protein aggregation while ensuring optimal functionality.

Affinity Chromatography
A cost-efficient platform for antibody production with comparable product quality to existing technologies and flexible for single use manufacturing.





