GORE® Protein Capture Devices with Protein A
Designed to improve productivity in protein A affinity purification of monoclonal antibodies, GORE Protein Capture Devices provide high binding capacity at short residence time.
Contact Us
Americas
+1 800 294 4673
+1 410 506 1715
Europe
+49 89 4612 3456
+800 4612 3456
US
+1 800 294 4673
+1 410 506 1715
Overview

GORE Protein Capture Devices in 1.0 mL, 3.5 mL, 9 mL, 58 mL, 116 mL, 232 mL, 250 mL, 500 mL and 1L sizes.
Traditional affinity chromatography technology has not kept pace with increased titers and other upstream process improvements, leading to bottlenecks and lower productivity in downstream protein A purification. To shorten processing times, traditional resin-based protein A is often over-sized or underutilized – simply to keep up.*
*Reference: “Disrupting Downstream Process Bottlenecks.” Genetic Engineering, June 14, 2018.
A cost-efficient platform for antibody production with comparable product quality to existing technologies. A flexible platform for single use manufacturing.
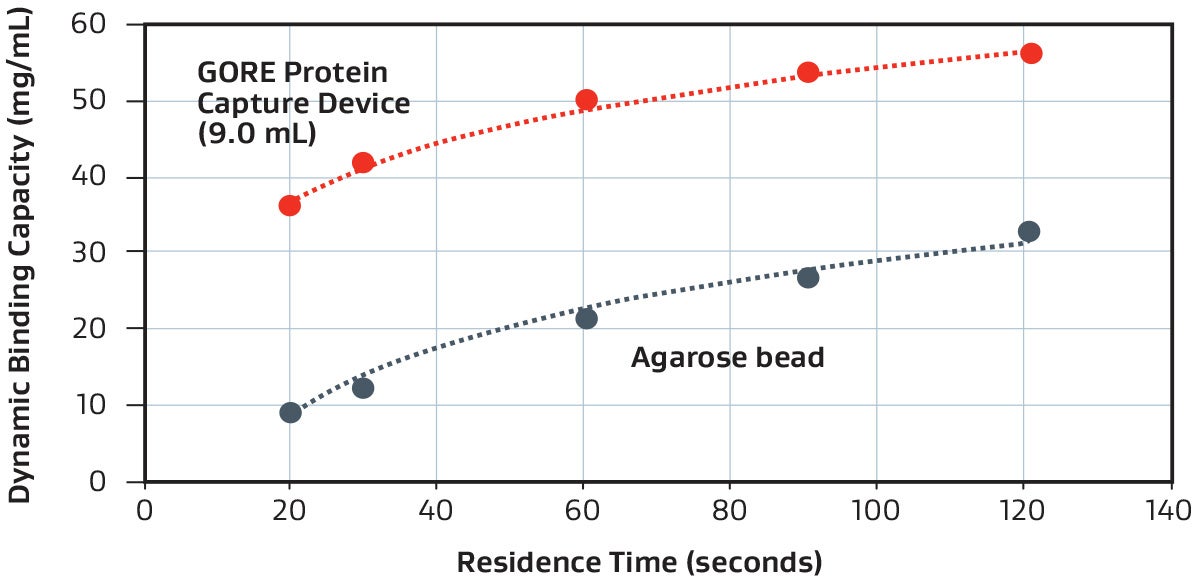
Representative dynamic binding capacity demonstrated with a 9 mL GORE Device (PROA103) versus a standard agarose bead-based technology, using a purified monoclonal antibody.
GORE Protein Capture Devices are approximately 6–10x faster than traditional (agarose bead-based) chromatography methods, which operate at typical residence times of 3–5 minutes (180–300 seconds).
Applications
Designed for affinity purification in early drug discovery screening, late stage optimization, process development, clinical and routine bioprocessing for
- monoclonal antibodies (mAbs)
- next-generation molecules that utilize the Fc region for Protein A binding
The columns are compatible with standard chromatography systems.
| Part Number | Description | Applications |
|---|---|---|
| PROA101 | 1.0 mL Device | Drug discovery |
| PROA102 | 3.5 mL Device | Drug discovery |
| PROA103 | 9.0 mL Device | Drug discovery, late stage optimization, process development, early GMP applications |
| PROA201 | 58 mL Device | Process development, clinical (GMP) applications |
| PROA202 | 116 mL Device | Clinical (GMP) applications |
| PROA203 | 232 mL Device | Clinical and Routine Manufacturing (GMP) applications |
| PROA301 | 250 mL Device | Clinical and Routine Manufacturing (GMP) applications |
| PROA302 | 500 mL Device | Clinical and Routine Manufacturing (GMP) applications |
| PROA303 | 1 L Device | Clinical and Routine Manufacturing (GMP) applications |
Performance
GORE Protein Capture Devices with immobilized Protein A* can improve throughput and yields in downstream purification by reducing overall process time through a combination of increased binding capacity and fast flow rates. These pre-packed columns use a unique membrane that offers high dynamic binding capacity at short residence time. Designed to increase the efficiency of protein A column purification processes, the Devices accommodate rapid cycle capability at low pressure drop, along with demonstrated ability to withstand multiple cleaning cycles. This increased productivity offers researchers the potential for a faster path to clinical trials.
| GORE Protein Capture Devices with Protein A (all sizes) | ||
|---|---|---|
| Dynamic binding capacity | ≥ 30 mg/mL | ≥ 40 mg/mL |
| Residence time | 20 seconds | 30 seconds |
| Number of cleaning cycles | 0.2 N NaOH for 3 minutes up to 100 cycles | 0.2 N NaOH for 3 minutes up to 100 cycles |
*recombinant Protein A
The GORE Protein Capture Device scales across sizes without compromising binding capacity or speed to quickly and effectively capture antibodies as shown in this overlay of a 1 mL, 9 mL and 58 mL bind-elute curve and dynamic binding capacity graph.
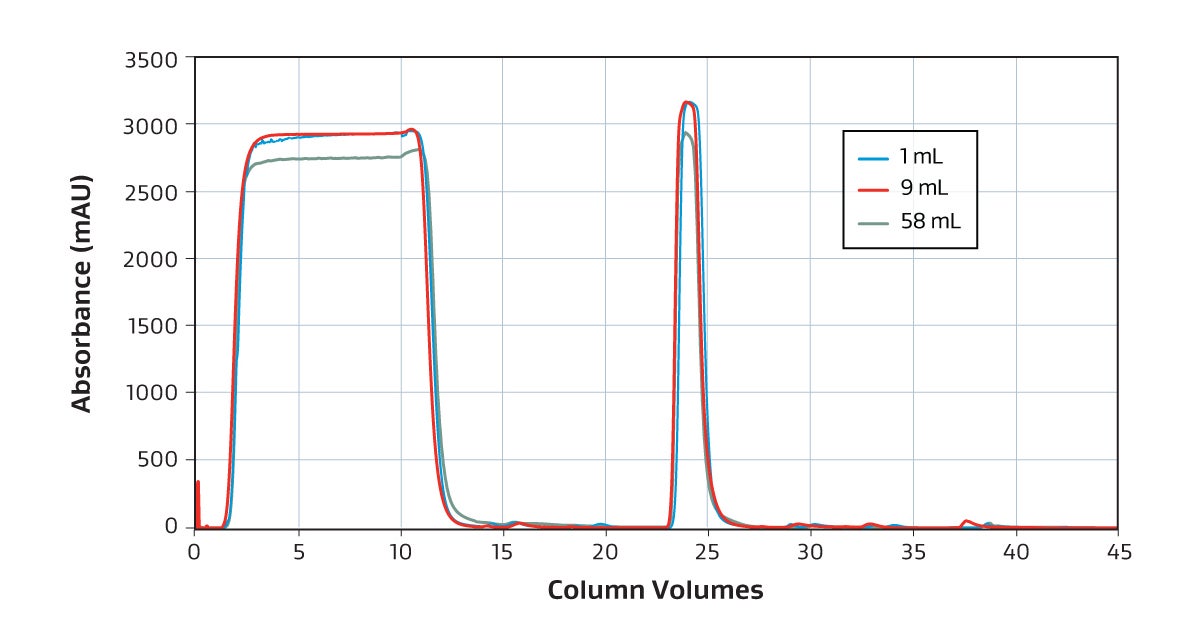
Overlaid chromatogram showing 1 mL (blue), 9 mL (red) and 58 mL (green) UV absorbance. The difference in UV of harvest reflects different LC instruments.
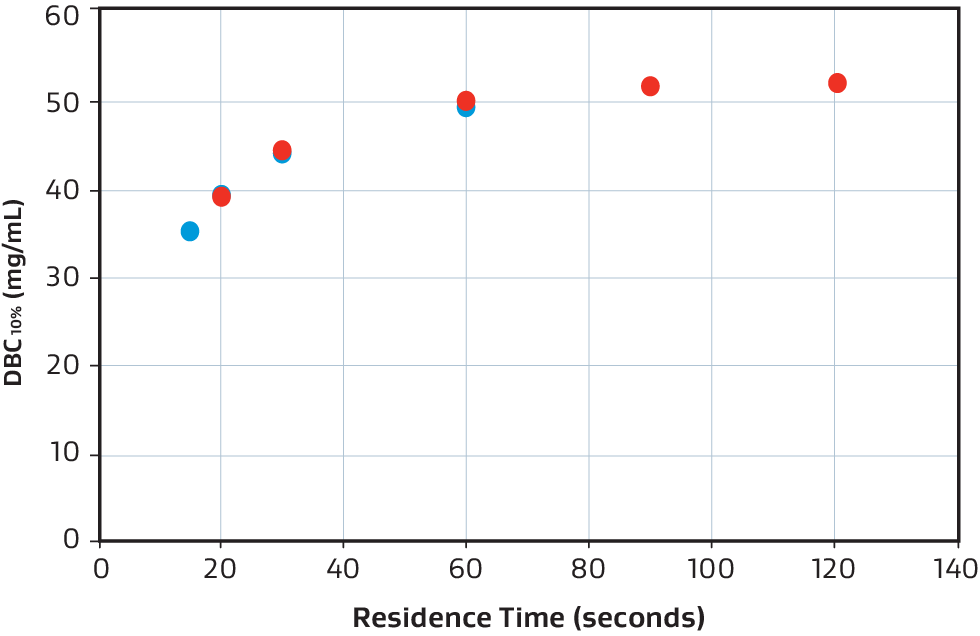
Overlaid DBC10% comparison of 58 mL (red) and 250 mL (blue).
Want complete technical and product performance information, including detailed test parameters, additional yield data, purity profiles and operating conditions?
Technology
The GORE Protein Capture Devices with Protein A can streamline antibody purification processes because they incorporate a proprietary membrane composite.
Unlike traditional support matrices, the composite membrane bed maintains a linear relationship between pressure drop and a wide range of flow rates. Because this unique structure remains stable, the membrane bed is not vulnerable to collapse, channeling or alteration of the bed.
Performance under pressure
Pressure Drop for a 58 mL Device (PROA201)
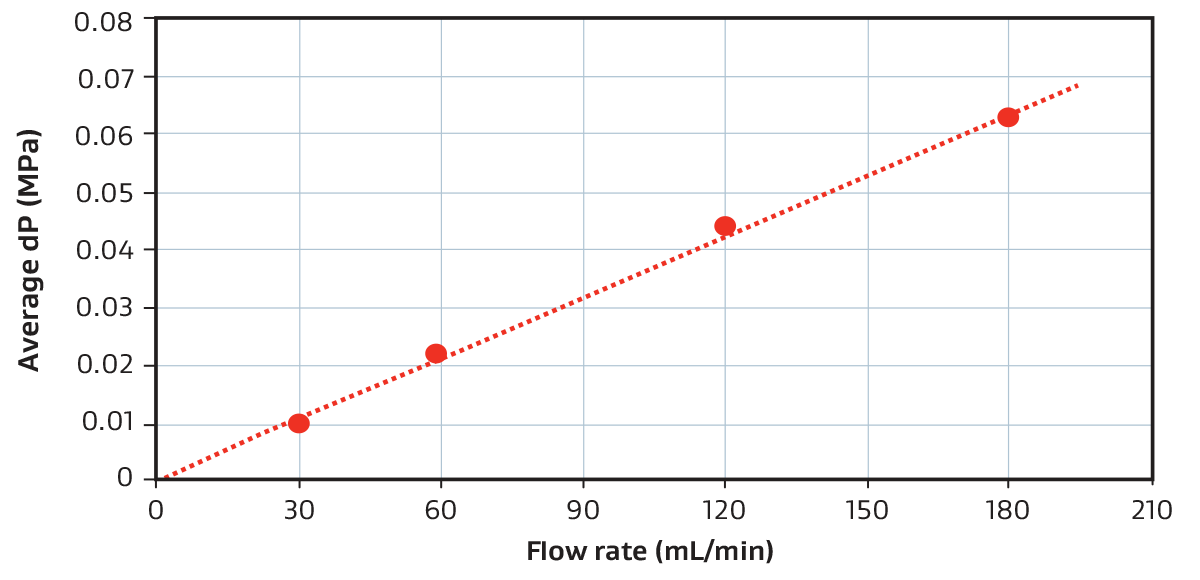
Example pressure drop vs. flow rate relationship for a GORE Device when using phosphate buffer at room temperature. For reference, 116 mL/min equates to 30 seconds residence time.
Pressure drop consistency
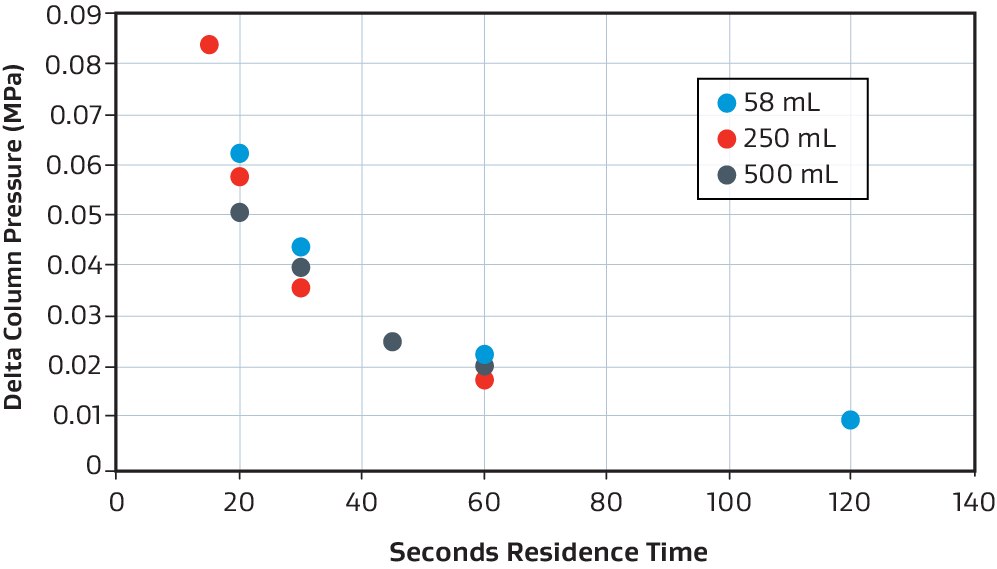
Example of pressure drop vs. residence time consistency for the 58 mL (blue), 250 mL (red), and 500 mL (grey) when using phosphate buffer at room temperature.
Materials of construction
| Components | GORE PART NUMBER | ||
|---|---|---|---|
| PROA101 PROA102 PROA103 |
PROA201 PROA301 |
PROA202 PROA203 PROA302 PROA303 |
|
| Device housing components | Polypropylene | Polypropylene and Polyether ether ketone (PEEK) primarily* | |
| Frit material(s) | Polypropylene | Polyether ether ketone (PEEK) Polyvinylidene fluoride (PVDF) |
|
| Membrane support matrix | Proprietary composite | ||
| Ligand | Recombinant Protein A | ||
| Connectors | 10-32 threaded fittings (PROA101/PROA102) 10-32 coned thread port (PROA103) |
5/16-24 Flat-bottom fittings (PROA201) 1/2-20 flat-bottom threaded fittings (PROA301) |
3/4" Tri clamp (0.984" (OD)) |
Availability
GORE Protein Capture Devices are compatible with standard pre-packed column chromatography systems, and are available in sizes as listed in table below. Contact Gore for pricing and availability. These Devices for protein A purification are not available in all regions.
Part Number/Ordering Information
| Part Number | Description |
| PROA101 | 1.0 mL Device |
| PROA102 | 3.5 mL Device |
| PROA103 | 9.0 mL Device |
| PROA201 | 58 mL Device |
| PROA202 | 116 mL Device |
| PROA203 | 232 mL Device |
| PROA301 | 250 mL Device |
| PROA302 | 500 mL Device |
| PROA303 | 1 L Device |
See Validation Guides for performance specifications and evaluations.
Regulatory Compliance and Quality
GORE Protein Capture Devices are manufactured following the applicable material quality and regulatory requirements, including relevant Good Manufacturing Processes as defined in the Gore PharmBIO quality system which is certified to ISO 13485 and ISO 15378. For current applicable compliance statements and quality control information:
Resources

Application Note: Cleaning Protocols to Reduce Effects of Pressure Rise Over the Lifetime of a GORE® Protein Capture Devices
Technical Information, 471.19 KB
Data Sheet: GORE Protein Capture Device for Early Clinical Applications, 9.0mL (PROA103)
Data Sheets, 245.48 KB

Data Sheet: GORE® Protein Capture Devices
Data Sheets, 212.86 KB
Recent News
We’re excited to announce our participation as the official Digital Partner at BioTalk EU 2024, the premier annual event where the world’s top pharma and biopharma manufacturing leaders converge. The exclusive, invitation-only conference is held in Berlin September 9-10 and serves as the gateway to groundbreaking innovations and industry-shaping discussions.
Event
Gore PharmBIO showcased our latest innovations at the Bioprocessing Summit 2024
August 19, 2024 - August 22, 2024
The Gore PharmBIO team attended the Boston event, exploring opportunities for collaboration with industry leaders. We shared our latest innovations designed to support and enhance your bioprocessing projects.
Event
Join W. L. Gore & Associates for the Live + Virtual Bioprocessing Summit Europe
March 19, 2024 - March 21, 2024
Join W. L. Gore & Associates at BioProcessing Summit Europe, Europe’s Premier Conference to Optimize Your Bioprocesses.
Event
Unpacking the Resin Alternatives: Single-Use Protein a Tech That Can Reduce Costs and Accelerate Your Clinical Journey
March 14, 2024
Explore exclusive research and analysis on the future of protein A purification, the potential of single-use protein A membrane capture devices and the opportunities they offer over costly resin columns during antibody drug development.
NOT INTENDED FOR USE in medical device or food contact applications or with radiation sterilization.


Follow GORE® Protein Capture Devices with Protein A